Peptides That Talk to Fat Cells:
The New Frontier of Adipose Tissue Signaling
How adipose tissue acts as an endocrine organ — and why peptides that influence fat-cell signaling are redefining metabolic research.
Research Disclaimer: This content is provided for educational and informational purposes only and reflects theoretical and ongoing research related to peptides, adipose tissue biology, and metabolic signaling pathways. This material does not constitute medical advice and is not intended for human or animal use.
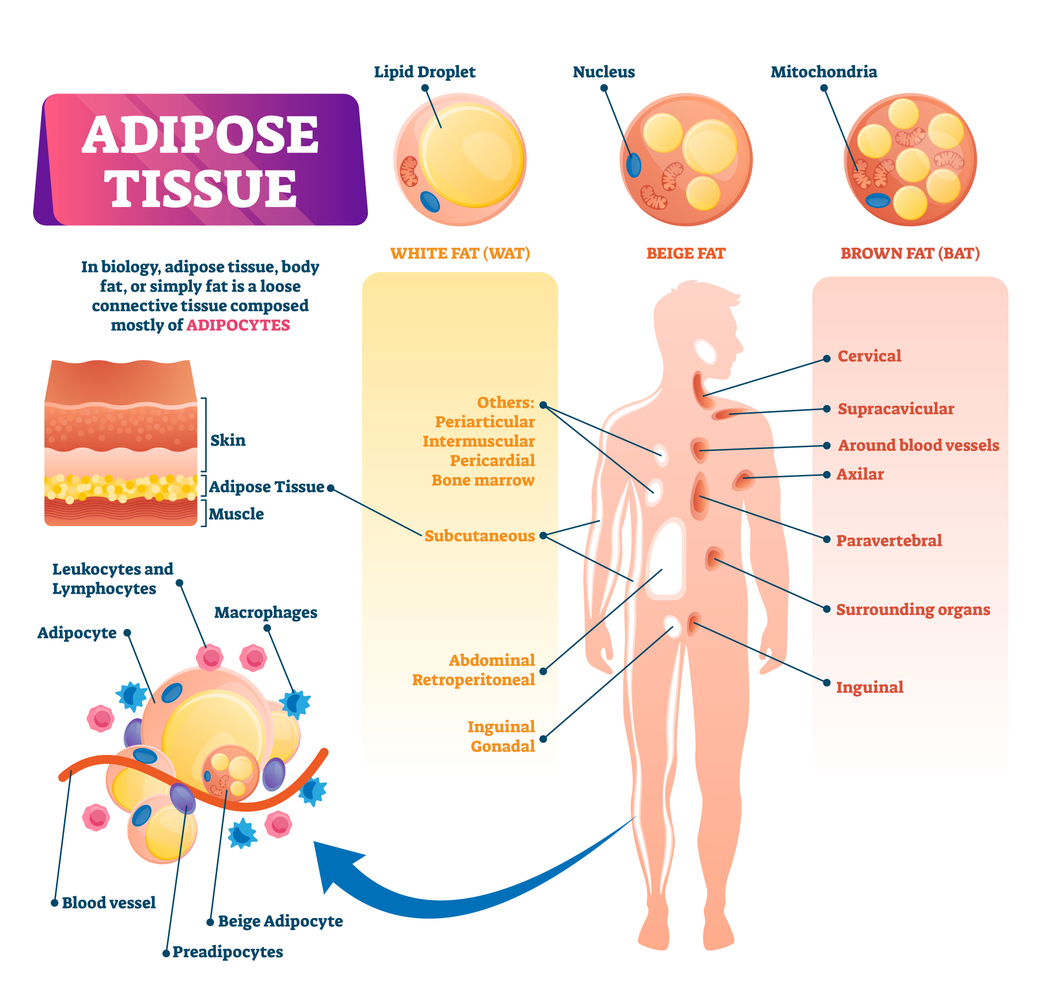
Adipose Tissue Is Not Passive Fat — It’s a Signaling Organ
For decades, body fat was viewed primarily as an inert storage depot for excess energy. Modern research has overturned that assumption entirely. Adipose tissue is now recognized as a dynamic endocrine and signaling organ that actively communicates with the brain, liver, muscle, pancreas, and immune system.
Fat cells (adipocytes) release signaling molecules known as adipokines, which influence appetite regulation, insulin sensitivity, inflammation, mitochondrial activity, and overall metabolic flexibility.

What Does It Mean for Peptides to “Talk” to Fat Cells?
In the context of adipose biology, peptides do not simply promote fat loss or fat gain. Instead, they influence how fat tissue behaves, communicates, and adapts.
Research indicates that certain peptides interact with adipose tissue by:
- Modulating adipokine secretion
- Influencing insulin and glucose signaling within fat cells
- Shifting fat tissue between storage and energy-expenditure modes
- Enhancing mitochondrial activity within adipocytes
This reframes adipose tissue as a programmable system — one that responds to signaling context, not just caloric surplus or deficit.
GLP-1 Pathways: More Than Appetite Signaling
Glucagon-like peptide-1 (GLP-1) is widely discussed in the context of appetite and glucose regulation, but adipose tissue expresses GLP-1 receptors as well.
Research suggests GLP-1 signaling may influence:
- Adipocyte insulin sensitivity
- Fat-cell lipid handling and storage behavior
- Cross-talk between adipose tissue and the central nervous system
Rather than simply reducing caloric intake, GLP-1-related signaling appears to reshape how fat tissue responds to energy availability — an important distinction in metabolic research.
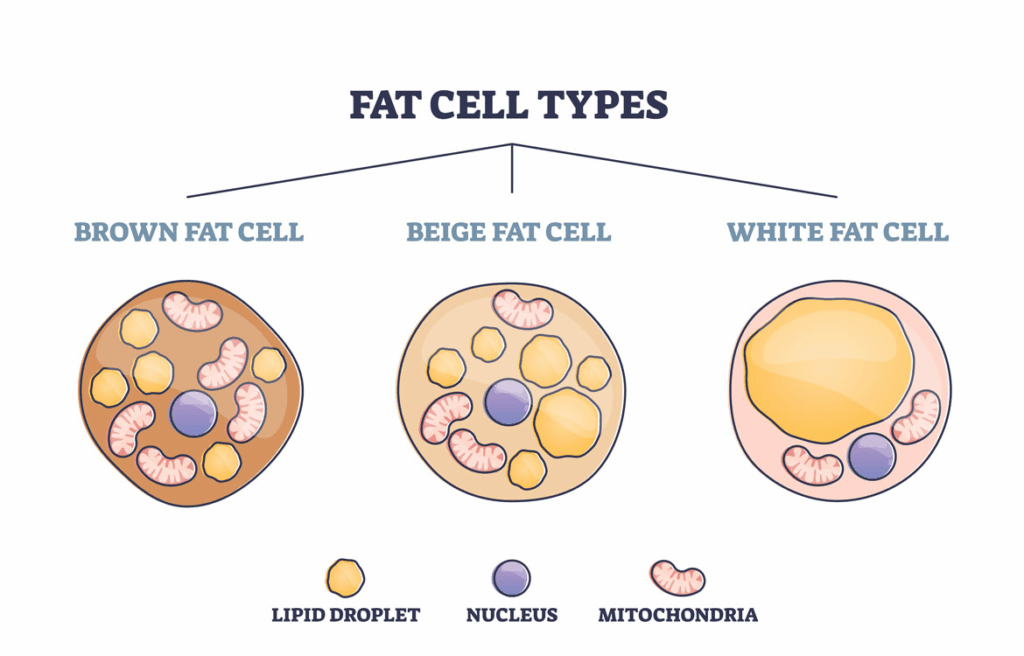
GIP and Brown Fat Activation
Glucose-dependent insulinotropic polypeptide (GIP) has historically been associated with insulin release, but newer research highlights its potential role in adipose tissue differentiation and energy expenditure.
Of particular interest is GIP’s interaction with brown adipose tissue (BAT) — a specialized form of fat designed for heat generation and energy dissipation rather than storage.
Brown fat contains a high density of mitochondria and expresses uncoupling protein-1 (UCP-1), allowing it to convert stored energy directly into heat.
Research exploring GIP signaling suggests it may influence:
- Brown fat activation and thermogenic potential
- Beige fat recruitment within white adipose depots
- Fuel partitioning between storage and expenditure
MOTS-C and Metabolic Flexibility
MOTS-C is a mitochondrial-derived peptide studied for its role in metabolic stress signaling. Unlike many peptides that act through surface receptors, MOTS-C appears to influence intracellular metabolic pathways directly.
In adipose tissue research, MOTS-C is frequently discussed in relation to:
- AMPK activation
- Improved metabolic flexibility
- Enhanced fat-cell responsiveness to energetic stress
Metabolic flexibility refers to the cell’s ability to switch efficiently between fuel sources — such as glucose and fatty acids — depending on environmental conditions. Dysfunctional adipose tissue often loses this flexibility.
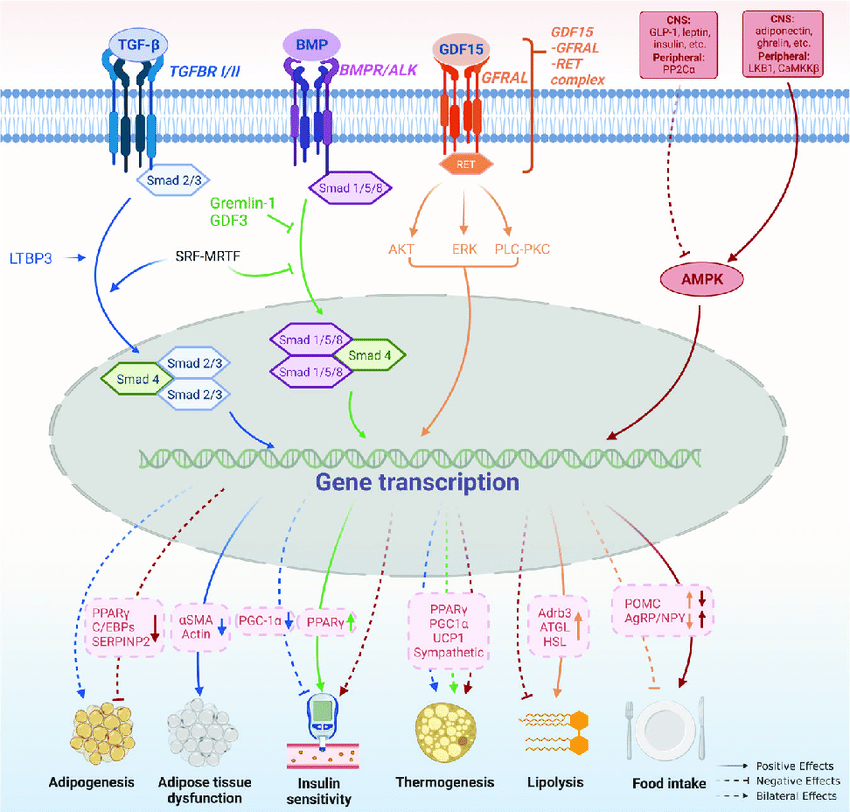
Adiponectin and AMPK: The Central Metabolic Axis
Adiponectin is one of the most studied adipokines released by fat cells. It plays a key role in insulin sensitivity, inflammation modulation, and mitochondrial activity across multiple tissues.
Adiponectin signaling strongly intersects with the AMPK pathway — a master regulator of cellular energy balance.
Peptides that influence AMPK activity, directly or indirectly, are therefore of significant interest in adipose tissue research, particularly in studies focused on metabolic resilience and longevity.
Why Adipose Signaling Matters More Than Fat Loss
Reducing fat mass alone does not necessarily restore metabolic health. Dysfunctional adipose tissue can remain inflamed, insulin-resistant, and metabolically rigid even after weight reduction.
Research increasingly emphasizes adipose quality over adipose quantity. Peptides that influence adipokine signaling, mitochondrial density, and metabolic flexibility may help explain why some metabolic interventions outperform others despite similar changes in body composition.
The Future of Adipose-Focused Peptide Research
Adipose tissue is no longer viewed as a passive bystander in metabolism. It is an active signaling hub — one that responds dynamically to peptides, hormones, nutrients, and environmental stress.
As research continues, peptides that “talk” to fat cells may define the next generation of metabolic science, shifting focus from simple calorie balance to cellular communication and adaptability.

RETATRUTIDE (GLP-3) 20mg
Retatrutide is an investigational multi-agonist peptide engineered to activate three metabolic hormone receptors—GLP-1, GIP, and glucagon—positioning it as a next-generation “triple agonist.” In research settings, it has been examined for its potential influence on appetite regulation, energy utilization, and body-weight–related endpoints. Chemically, retatrutide is a long-acting incretin-mimetic designed to co-activate GLP-1R, GIPR, and GCGR, enabling coordinated engagement of complementary metabolic pathways. Studies have explored its combined effects on glucose homeostasis, gastric emptying, insulin dynamics, lipolysis, and energy expenditure within controlled experimental models.
In Stock