The Peptide “Second Brain”: How Gut-Signaling Peptides Influence Mood, Immunity & Metabolism
Research & Education Disclaimer: This article is for research and educational discussion only. It does not constitute medical advice, diagnosis, or treatment. Any compounds mentioned are intended strictly for controlled laboratory research and analytical use only and are not for human or animal consumption, injection, or therapy.
Introduction
The most exciting breakthroughs in peptide research are not just happening in muscles, fat cells, or even the brain—they are happening in the gut.
The gut is more than a digestive organ. It is a neuroendocrine signaling hub that communicates continuously with the brain, immune system, adipose tissue, liver, and endocrine system. And peptides are among the primary messengers coordinating this flow of information.
Understanding gut peptide signaling is becoming essential for research on metabolism, mood, inflammation, recovery, and healthy aging.
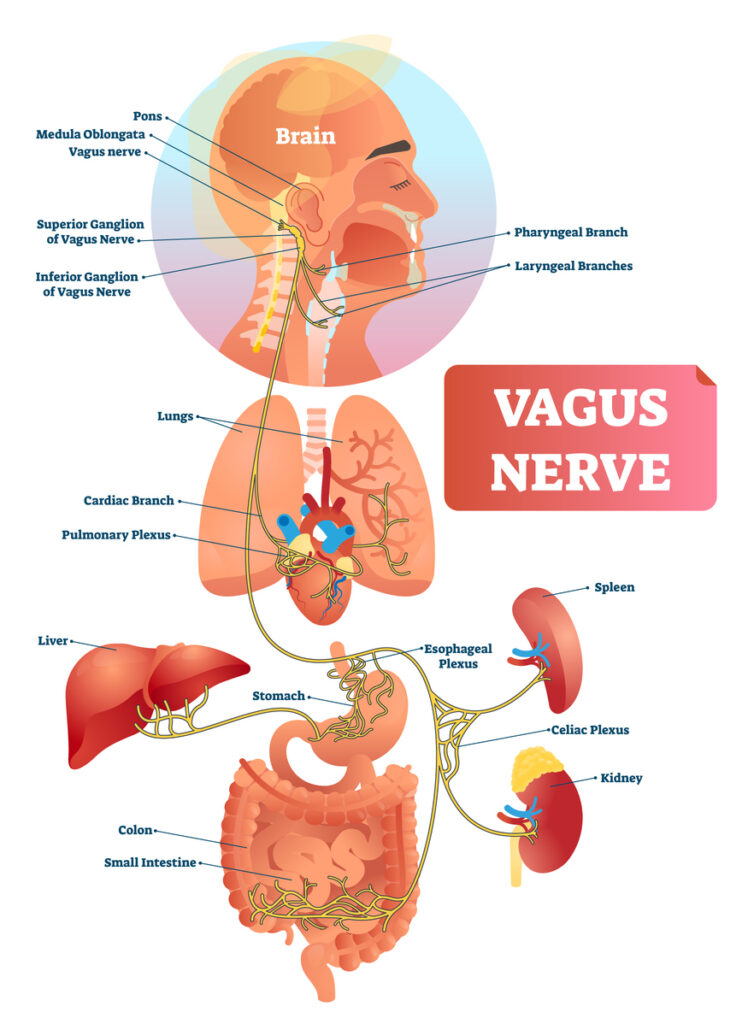
Section 1: The Gut–Brain Axis — A Peptide Superhighway
The gut contains the enteric nervous system (ENS), sometimes called the “microbrain.” It houses more neurons than the spinal cord and produces a range of bioactive signals, including neurotransmitters, hormones, peptides, and cytokines.
Several key communication routes link the gut to the brain and the rest of the body:
1. Vagal Signaling
The vagus nerve sends rapid, real-time information from the gut to the brainstem and limbic system. Peptides that alter gut motility, inflammation, or hormone release can modulate:
- Satiety perception
- Reward and craving behavior
- Stress responses
- Emotional tone and arousal
2. Hormonal (Endocrine) Signaling
Peptides such as GLP-1, GIP, PYY, and oxyntomodulin are released from intestinal L-cells and other enteroendocrine cells. They enter circulation and influence:
- Appetite and meal size
- Blood glucose regulation
- Insulin sensitivity
- Energy expenditure and fat oxidation
- Reward loops linked to food and comfort behaviors
3. Immune and Cytokine Signaling
The gut houses a large portion of the immune system. When gut inflammation rises, pro-inflammatory cytokines can influence the brain’s immune cells (microglia), impacting mood, cognition, and stress sensitivity.
4. Microbiome–Peptide Interactions
Gut bacteria produce bioactive molecules and peptide-like metabolites that can modulate local hormone and peptide signaling. This means that peptide research can intersect with microbiome-dependent pathways in complex ways.
Together, these four routes create a peptide superhighway between gut and brain, where changes in gut peptide signaling can have system-wide effects.
Section 2: Mood, Stress & Cognitive Pathways — Peptides Starting in the Gut
Many of the most interesting mood and stress effects observed in peptide research may be driven less by direct brain exposure and more by gut-origin signals that travel upward.
GLP-1, GIP & Glucagon Co-Agonists (Examples: Semaglutide-, Tirzepatide-, Retatrutide-Class Peptides)
GLP-1-based and multi-agonist peptides are primarily studied in the context of metabolic and bodyweight research, but they originate in and signal through the gut before influencing the brain.
Key research mechanisms include:
- Activation of receptors on vagal afferent neurons in the gut wall
- Signaling to the nucleus tractus solitarius (NTS) and hypothalamic appetite centers
- Modulation of dopaminergic reward circuits associated with cravings and impulsive eating
- Reduction of local intestinal inflammation that can disturb serotonin and immune balance
These gut-origin signals may help explain why research often notes not only appetite changes but also shifts in emotional relationship with food, reduced compulsive tendencies, and calmer decision-making around eating.
MOTS-C — A Mitochondrial Peptide with Strong Gut Relevance
MOTS-C is a mitochondrial-derived peptide encoded in mitochondrial DNA (mtDNA). It is expressed in several tissues but has particularly high relevance in metabolically active cells, including those of the gastrointestinal tract.
Within gut tissue, MOTS-C has been explored for its potential to:
- Activate AMPK and related energy-sensing pathways
- Improve cellular handling of glucose and fatty acids
- Reduce oxidative stress and mitochondrial dysfunction
- Support more stable metabolic responses to dietary and environmental stressors
By supporting healthier mitochondria in gut cells, MOTS-C research may intersect with reductions in neuroinflammatory signaling and improvements in stress tolerance and mental energy.
BPC-157 — Bridging Gut Integrity & Serotonin Dynamics
BPC-157 is a peptide fragment originally isolated from gastric juice. It has been widely studied in preclinical models for its potential roles in tissue protection and repair, particularly within the gastrointestinal tract.
Research suggests possible actions such as:
- Support of the mucosal lining and epithelial restitution
- Influence on tight junction proteins that maintain gut barrier integrity
- Modulation of local neurotransmitter systems, including serotonin transport
- Regulation of nitric oxide (NO) pathways within the enteric nervous system
Since the majority of the body’s serotonin is produced in the gut rather than the brain, improvements in gut integrity and local signaling may have downstream implications for mood, emotional stability, and cognitive clarity in research contexts.

Section 3: Immune Modulation Through Gut Peptides
Approximately 70% of the immune system resides in and around the gut. Peptides here do not just regulate digestion—they help decide whether the immune system is calm and tolerant or hyperactive and inflamed.
Thymalin and Other Thymic Peptides
Thymalin and related thymic peptides have been studied for decades as modulators of immune function. They appear to influence:
- T-cell maturation and differentiation
- Normalization of T-helper / T-suppressor cell ratios
- Balancing of cytokine patterns, including key inflammatory mediators
When immune balance is improved system-wide, the gut can respond more appropriately to food antigens, microbes, and stressors, reducing inflammatory noise that would otherwise echo into the nervous system.
KPV (α-MSH Fragment) and MC1R Pathways
KPV is a tripeptide fragment derived from alpha-melanocyte-stimulating hormone (α-MSH). It interacts with melanocortin receptors such as MC1R, which are expressed in skin and also in the gastrointestinal tract.
Research suggests that KPV may:
- Suppress excessive pro-inflammatory cytokines in mucosal tissues
- Stabilize mast cells and reduce histamine-driven flare-ups
- Influence nerve hypersensitivity patterns in the gut
- Support more robust barrier function under stress
By helping calm immune overactivation in the gut lining, KPV-related pathways may indirectly reduce systemic inflammatory burden and downstream brain inflammation.
Peptides and Gut Barrier Integrity
The gut barrier is controlled by tight junction proteins such as occludin, claudins, and ZO-1. When these proteins are disrupted, intestinal permeability (“leaky gut”) increases, allowing bacterial components and toxins to enter circulation and trigger inflammation elsewhere.
Peptides that have been studied in the context of barrier integrity include:
- BPC-157 – GI mucosal and vascular support in preclinical models
- KPV – Inflammatory control at the mucosal level
- Thymic peptides – Systemic immune normalization that indirectly supports barrier resilience
Maintaining healthier barrier function may help reduce the passage of inflammatory triggers that can ultimately contribute to fatigue, brain fog, and mood disturbances.
Section 4: Metabolic Control via Gut Peptide Signaling
The gut is the command center for appetite, energy use, and metabolic adaptation. Many metabolic peptides are, at their core, gut-derived signals.
PYY (Peptide YY)
PYY is secreted by L-cells in the ileum and colon in response to nutrient intake. It binds to Y2 receptors and plays an important role in:
- Promoting satiety and reducing meal size
- Signaling through the vagus nerve to appetite centers
- Reducing hedonic, reward-driven overeating
- Potentially improving insulin sensitivity in research settings
PYY-focused research is expanding as investigators explore next-generation satiety and metabolic peptides.
Oxyntomodulin and Multi-Agonist Metabolic Peptides
Oxyntomodulin and related peptides can activate multiple receptors, including GLP-1 and glucagon receptors. The result is a multi-axis signal that can influence:
- Energy expenditure and thermogenesis
- Lipolysis and fat oxidation
- Brown adipose tissue activity
- Appetite suppression and meal frequency
These multi-agonist enteroendocrine peptides represent a new wave of metabolic research with both gut and central effects.
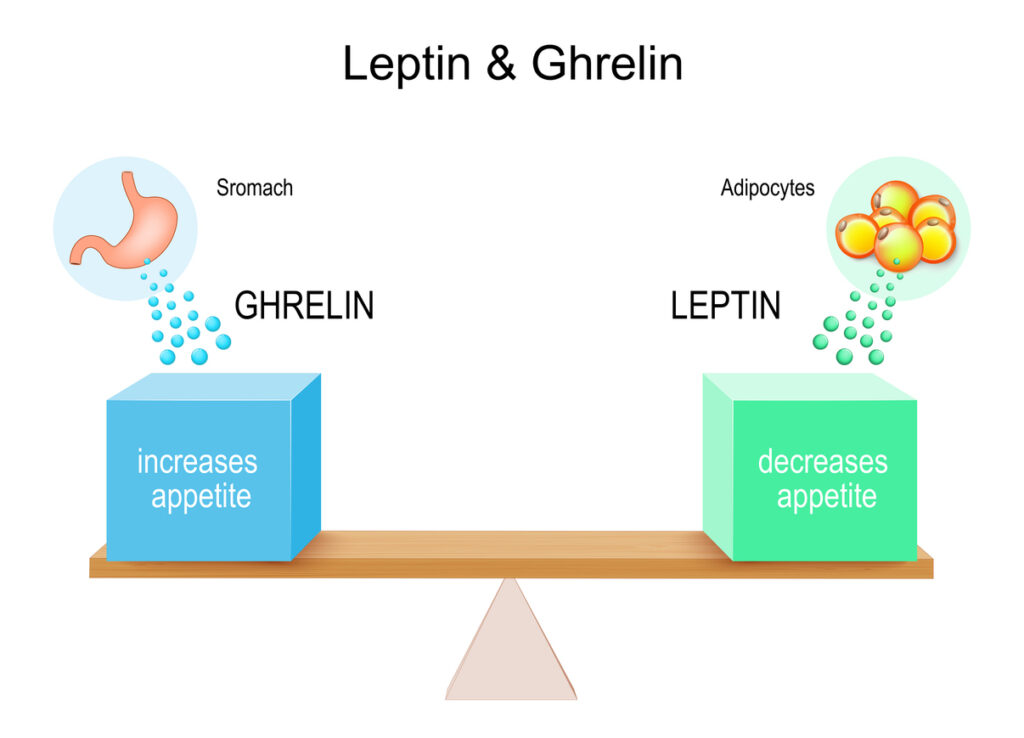
Ghrelin Pathway Modulators
Ghrelin is often called the “hunger hormone,” but it is more accurately a meal-timing and stress-response hormone produced primarily in the stomach.
Peptides that interact with ghrelin pathways, including certain growth hormone secretagogues (theoretical discussion only), can influence:
- Hunger timing and intensity
- Reward circuits related to food and comfort-seeking
- Circadian feeding cycles
- Perceived stress and resilience
Section 5: Neuroinflammation and Peptide Modulation
Chronic gut inflammation can promote systemic inflammation and activate immune cells in the brain known as microglia. When microglia become overactive, they can contribute to:
- Heightened anxiety and stress sensitivity
- Cognitive fog and slowed processing
- Increased pain perception
- Disrupted sleep and mood cycles
Peptides investigated in the context of this gut–brain inflammatory loop include:
- MOTS-C – Supporting mitochondrial resilience and reducing oxidative stress
- BPC-157 – Supporting mucosal and vascular integrity under stress
- KPV – Modulating inflammatory pathways in mucosal tissues
- Thymic peptides – Normalizing immune activation patterns
By cooling the inflammatory “noise” at the gut level, these pathways may contribute to calmer systemic and neurological environments in research models.
Section 6: Theoretical Research Stack Concepts (For Lab Discussion Only)
Important: The following are theoretical research stack concepts, not recommendations for use. They are provided purely as examples of how different peptide pathways could be studied together in controlled, compliant laboratory settings.
1. Gut Repair + Serotonin Balance (Theoretical)
- BPC-157 – GI lining and vascular support in preclinical models
- KPV – Mucosal anti-inflammatory and barrier-supporting pathways
- Thymalin – Systemic immune modulation and cytokine balance
2. Metabolic Axis Enhancement (Theoretical)
- GLP-1 / GIP / glucagon-type peptide – Appetite, glucose, and energy expenditure in research
- MOTS-C – Mitochondrial and metabolic resilience
- PYY-pathway peptide – Satiety and hedonic intake control
3. Stress & Reward Circuit Modulation (Theoretical)
- Ghrelin-pathway peptide – Meal timing and reward signal modulation
- GLP-1-type peptide – Dopamine and reward-circuit modulation in appetite research
4. Gut Barrier & Immune Reset (Theoretical)
- Thymalin – T-cell and cytokine pattern support
- KPV – Local inflammatory tone modulation
- BPC-157 – Epithelial and vascular integrity support
These conceptual frameworks reflect how deeply connected gut peptides are to mood, metabolic, and immune research domains.

Section 7: Why the Gut–Brain Peptide Axis Matters for Modern Research
When research focuses only on a single endpoint—such as joint tissue, skin quality, or body weight—it can miss how strongly the gut–brain axis ties everything together. Gut-signaling peptides form a common thread between:
- Metabolic regulation and appetite
- Immune balance and inflammation
- Mood, focus, and emotional regulation
- Recovery from physical and metabolic stress
Viewing the gut as a “second brain” is not just a metaphor; it is a practical framework for designing more integrated, multi-axis research protocols. Peptide signaling sits at the center of this framework.
For advanced peptide researchers, the gut is emerging as a primary control node. Understanding gut-driven peptide pathways can open the door to more nuanced, holistic studies that respect the complexity of human physiology.
BioGenix Peptides Notice: BioGenix Peptides LLC supplies products strictly for research, laboratory, and analytical use. Products are not intended for human or animal consumption, or for any diagnostic, therapeutic, or cosmetic use. BioGenix Peptides LLC is not a compounding pharmacy or medical provider. No statements in this article have been evaluated by the U.S. Food and Drug Administration, and nothing herein is intended to diagnose, treat, cure, or prevent any disease.