New Enzyme-Based Chemistry Is Making Peptides More Stable
Why Structural Optimization Is the Next Major Breakthrough in Peptide Research
Research Use Only Disclaimer
This content is provided for educational and informational purposes only and reflects theoretical and preclinical research discussions related to peptides, biochemistry, and molecular engineering. It does not constitute medical advice and is not intended for human or animal use.
Introduction: The Longstanding Stability Challenge in Peptide Research
Peptides are foundational tools in biochemical and molecular research. Their ability to interact with biological targets with high specificity makes them invaluable for studying signaling pathways, enzyme regulation, and receptor dynamics.
Despite these advantages, peptides have historically faced one major limitation: structural instability. Linear peptides are often rapidly degraded by proteolytic enzymes, structurally flexible in solution, and short-lived in biological environments. These challenges directly affect peptide bioavailability and molecular persistence, complicating experimental design and reducing reproducibility.
Recent advances in enzyme-guided peptide modification suggest that stability is increasingly becoming a controllable design parameter rather than an inherent limitation.
A Recent Breakthrough: Enzyme-Guided Peptide Cyclization
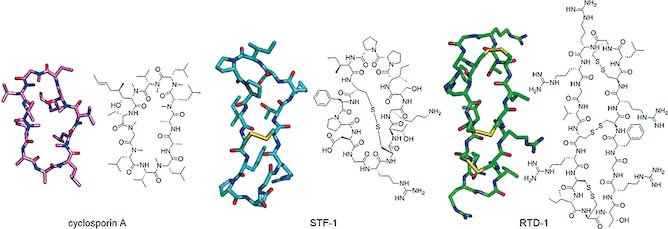
Recent research has identified naturally occurring enzymes capable of catalyzing precise peptide cyclization, transforming linear peptide chains into closed-loop, cyclic structures under mild biochemical conditions.
Unlike traditional chemical approaches, enzymatic macrocyclization occurs with high regioselectivity, preserves native peptide bonds or stable cross-links, and closely mirrors natural peptide maturation pathways. This represents a significant advance in biologically aligned peptide engineering.

Why Cyclic Peptides Behave Differently
Cyclization fundamentally alters peptide behavior by restricting conformational flexibility. This structural constraint reduces exposure to enzymatic cleavage sites and stabilizes bioactive conformations.
Within research systems, cyclic peptides commonly demonstrate improved resistance to degradation, greater structural persistence, and more consistent target engagement. These advantages have positioned macrocyclization as a peptide design strategy for researchers seeking predictable molecular behavior over extended study periods.

Enzymatic vs. Chemical Cyclization: Why the Distinction Matters
Chemical macrocyclization techniques have been used for decades, but they often involve non-native linkages, low yields, or limited sequence tolerance. In contrast, enzyme-mediated approaches provide high specificity, reproducibility, and biological relevance.
This form of enzyme-guided peptide modification aligns structural stabilization with laboratory best practices, preserving molecular integrity without introducing unnecessary chemical complexity.
Implications for Metabolic and Signaling Research
Many peptides studied in metabolic and signaling research require sustained structural integrity to accurately model receptor engagement, signal duration, and downstream pathway behavior.
By improving molecular persistence, stabilized cyclic peptides allow researchers to better study receptor activation, desensitization, and signaling duration without compensating for rapid degradation or loss of functional structure.
Bridging the Gap Between Peptides and Small Molecules
One notable implication of enzymatic cyclization is that stabilized peptides begin to exhibit small-molecule-like durability while retaining peptide-level specificity.
This hybrid behavior expands how peptides can be used to explore how peptides interact with intracellular targets, positioning them as precision molecular probes rather than transient signaling fragments.
A Broader Shift in Peptide Engineering Philosophy
This breakthrough reflects a larger shift in peptide science: innovation is increasingly focused on engineering peptide behavior rather than discovering new sequences alone.
Emerging research emphasizes enzyme-assisted folding, macrocyclization, and AI-assisted peptide structure optimization as tools for designing predictable, programmable peptide systems.
Final Thoughts
Enzyme-guided peptide stabilization represents a meaningful advance in molecular engineering, allowing peptides to function as durable and predictable research tools.
As peptide science continues to evolve, stability is no longer viewed as a limitation — it is becoming a deliberate design choice.
Related Peptide Research Articles
- Peptides & Blood–Brain Barrier Integrity: The Underexplored Frontier
- The “Peptide Reserve” Theory: Why the Body Responds Differently After Saturation vs. Depletion
- How Environmental Stress Changes Peptide Responsiveness
References (Peer-Reviewed Research)
- Bechtler, J., Waldmann, H., & Kruse, M. (2021). Macrocyclization strategies for cyclic peptides and peptidomimetics. Chemical Reviews, 121(4), 2210–2250.
https://pmc.ncbi.nlm.nih.gov/articles/PMC8372203/ - Fang, G., et al. (2024). Recent advances in peptide macrocyclization strategies. Chemical Society Reviews, 53, 1452–1483.
https://pubs.rsc.org/en/content/articlehtml/2024/cs/d3cs01066j - Schmidt, M., et al. (2017). Enzyme-catalyzed peptide cyclization. Biotechnology Advances, 35(3), 338–349.
https://www.sciencedirect.com/science/article/abs/pii/S174067491630049X - Eastman, A. W., et al. (2025). Diverse thioether macrocyclized peptides through a radical SAM maturase. Proceedings of the National Academy of Sciences, 122(9).
https://www.pnas.org/doi/10.1073/pnas.2512563122


