How Environmental Stress Changes Peptide Responsiveness:
Research Disclaimer: The following content is provided for informational and educational purposes only. It reflects theoretical and ongoing research discussions related to peptides, cellular stress biology, and biochemical signaling. This content does not constitute medical advice and is not intended for human or animal use.
The Stress-Adaptive Model
Why peptides behave differently under cold exposure, heat stress, and fasting — and what research is uncovering about cellular adaptability.
The Missing Variable in Peptide Research: Environmental Stress
Most peptide discussions focus on molecular structure, purity, dosing theory, or receptor binding. Far fewer address a critical variable that profoundly alters peptide behavior: the physiological state of the cell at the moment of exposure.
Emerging research suggests that peptides do not act in isolation — their signaling impact is heavily influenced by whether cells are under stress, adapting, or metabolically “comfortable.” This has led to what researchers increasingly describe as a stress-adaptive model of peptide responsiveness.
What Is the Stress-Adaptive Model?
The stress-adaptive model proposes that acute environmental stressors temporarily shift cellular priorities. Under these conditions, cells alter receptor sensitivity, mitochondrial signaling, and gene expression — creating a biochemical environment where certain peptides produce amplified or qualitatively different effects.
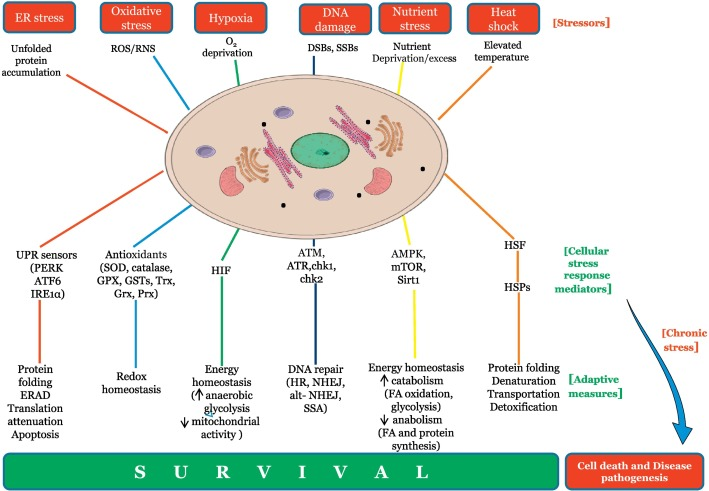
Cold Exposure: Mitochondrial Signaling Takes the Lead
Cold exposure represents a powerful acute stressor. Cells respond by increasing mitochondrial efficiency, activating AMPK, and shifting fuel utilization toward survival-oriented metabolism.
In this state, peptides involved in mitochondrial protection and metabolic resilience appear to operate within a more receptive signaling environment.
Key Research Peptides of Interest
- MOTS-C – Studied for its role in metabolic adaptation and AMPK activation
- SS-31 – Researched for cardiolipin binding and mitochondrial membrane stabilization
- Humanin – Examined for cytoprotective signaling under cellular stress

Heat Stress: Protein Protection and Cellular Repair
Heat exposure triggers the heat-shock response — a conserved protective mechanism designed to prevent protein misfolding and cellular damage.
During this phase, cells upregulate chaperone proteins, repair enzymes, and stress-responsive signaling networks. Peptides interacting with mitochondrial integrity and cytoprotection may exhibit altered signaling dynamics under these conditions.
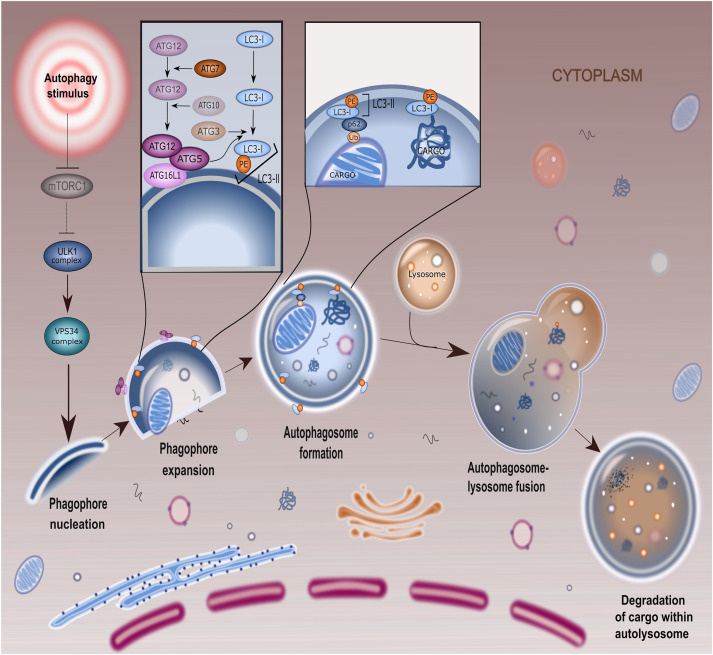
Fasting: Autophagy, AMPK, and Peptide Sensitivity
Fasting introduces a unique metabolic stress — one that suppresses growth signaling while amplifying repair, recycling, and mitochondrial renewal.
Research indicates that fasting alters receptor density and intracellular signaling thresholds, potentially changing how cells respond to metabolic and mitochondrial peptides.
Peptides Commonly Discussed in Fasting-Related Research
- MOTS-C – Metabolic stress signaling
- Humanin – Cell survival under nutrient deprivation
- 5-Amino-1MQ – Investigated for effects on NNMT-related metabolic pathways
Why This Matters for Peptide Research
The stress-adaptive model challenges the assumption that peptide responsiveness is static or predictable under all conditions. Instead, it suggests that context matters as much as compound.
Environmental stress may:
- Enhance signaling efficiency
- Alter receptor availability
- Shift cellular priorities toward repair and survival
This may help explain why identical peptides can produce dramatically different research observations across varying conditions.
Looking Ahead: A New Layer of Peptide Science
As peptide research continues to evolve, environmental context is emerging as a critical variable. Cold, heat, and fasting are not merely lifestyle factors — they are powerful biological switches that reshape how cells interpret peptide signals.
Understanding this interaction may define the next generation of peptide research strategies.

5-AMINO-1MQ 50mg
5-Amino-1MQ is a small-molecule research compound investigated for its effects on cellular energy balance and fat metabolism. Unlike retail capsule formulations, this overview pertains to the lyophilized powder presentation intended for controlled laboratory work. The compound is best known as an inhibitor of nicotinamide N‑methyltransferase (NNMT), an enzyme implicated in adipose tissue energy handling and nicotinamide adenine dinucleotide (NAD+) turnover.
In Stock