GLP-1 & Incretin Pathways Explained
Foundational Research Explainer
GLP-1 & Incretin Pathways Explained
A mechanism-first overview of incretin biology, exploring GLP-1, GIP, the gut–brain axis, glucose-dependent insulin signaling, and why modern research increasingly favors systems-level metabolic models. Educational Mechanism-Focused Research Context

Research Disclaimer: Research Use Only. This content is provided for educational and informational purposes only and discusses biochemical and physiological pathways described in the scientific literature. It does not constitute medical advice. All compounds referenced are intended strictly for laboratory, analytical, and research use only and are not for human or animal consumption.
Why Incretins Matter
Interest in GLP-1 biology has expanded rapidly in recent years, but GLP-1 does not operate in isolation. It belongs to a broader endocrine communication network known as the incretin pathway. This system links nutrient intake in the gut to coordinated hormonal responses across the pancreas, brain, and peripheral tissues.
Incretin signaling provides a biological explanation for how feeding state can influence insulin dynamics, appetite regulation, and metabolic efficiency through hormone-based communication rather than direct stimulation.
What Are Incretins?
Incretins are peptide hormones released from the gastrointestinal tract in response to nutrient exposure. Their defining feature is the ability to enhance insulin secretion in a glucose-dependent manner, linking digestion directly to metabolic signaling.
The two primary incretins studied in humans are:
- GLP-1 (Glucagon-Like Peptide-1)
- GIP (Glucose-Dependent Insulinotropic Polypeptide)

GLP-1: The Central Signal
GLP-1 is secreted primarily by L-cells in the distal small intestine and colon following nutrient intake, particularly carbohydrates and fats. Once released, GLP-1 interacts with receptors distributed across multiple systems.
- Pancreatic beta cells
- Central nervous system appetite-regulation centers
- Gastrointestinal tissue
- Cardiovascular tissue
This broad receptor distribution explains why GLP-1 signaling is studied across metabolic, neurological, and endocrine research domains.
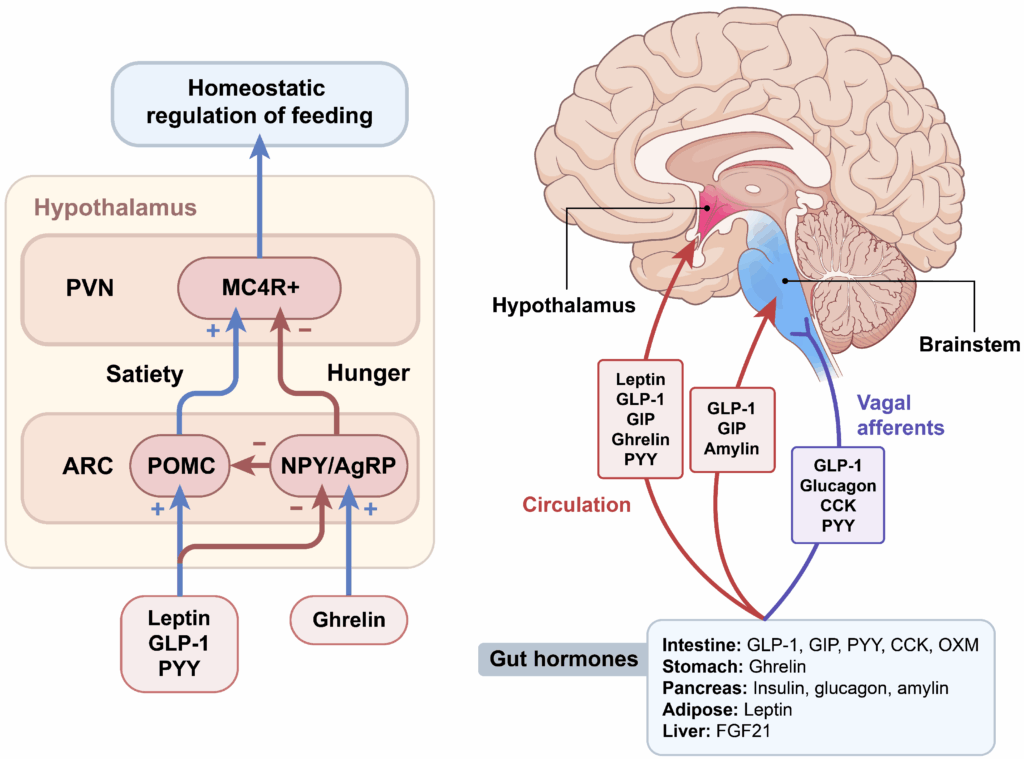
The Gut–Brain Axis
GLP-1 plays a significant role in the gut–brain axis, a bidirectional communication network linking the gastrointestinal tract with neural centers involved in appetite and feeding behavior.
Research discussions commonly associate GLP-1 signaling with increased satiety signaling, modulation of reward-driven feeding, and changes in food-seeking behavior.
These effects are typically described as neuroendocrine modulation rather than stimulant-driven appetite suppression.
GLP-1 and Glucose-Dependent Insulin Signaling
A defining characteristic of incretin biology is glucose-dependent insulin secretion. GLP-1 signaling amplifies insulin release when circulating glucose levels are elevated, making it a central topic in metabolic physiology research.
- Enhancement of insulin secretion under elevated glucose conditions
- Suppression of inappropriate glucagon signaling post-feeding
- Support of post-prandial glucose handling
GIP: The Complementary Incretin
GIP is secreted by K-cells in the proximal small intestine and functions as a complementary incretin hormone. Modern research increasingly emphasizes GIP’s role in adipocyte signaling, lipid handling, and metabolic flexibility.
Rather than acting redundantly, GLP-1 and GIP are now often discussed as synergistic components within integrated metabolic signaling models.
Beyond Single-Pathway Thinking
Early incretin research often focused on individual hormone effects. Contemporary models emphasize network-level interactions involving GLP-1, GIP, glucagon signaling, and downstream cellular energy pathways.
This systems-based view helps explain why modern research increasingly explores dual- and multi-agonist signaling frameworks.
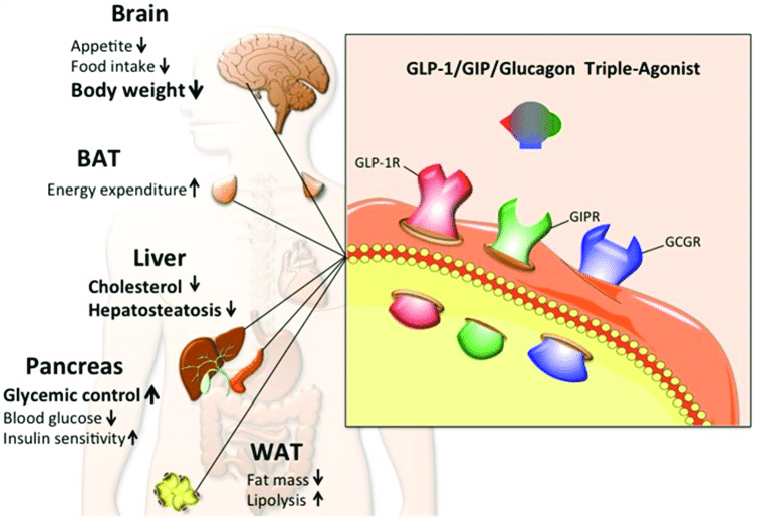
Incretins and Metabolic Efficiency
Incretin signaling is increasingly framed around metabolic efficiency—how organisms sense nutrient availability and align endocrine output with energy demands.
- Energy partitioning signals
- Nutrient sensing fidelity
- Coordination between feeding state and hormone release
Why GLP-1 Research Keeps Expanding
GLP-1 occupies a central position at the intersection of endocrinology, neurology, and metabolism. Its importance lies not in a single outcome, but in its role as a signaling coordinator translating gut-derived information into systemic responses.
Peer Reviewed References
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165.
https://pubmed.ncbi.nlm.nih.gov/16517405/ - Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21.
https://pubmed.ncbi.nlm.nih.gov/30253101/ - Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439.
https://pubmed.ncbi.nlm.nih.gov/17928588/ - Campbell JE, Drucker DJ. Islet α cells and glucagon—critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11:329–338.
https://pubmed.ncbi.nlm.nih.gov/25971867/ - StatPearls Publishing. Physiology, Incretin Hormones. StatPearls [Internet]. Treasure Island (FL).
https://www.ncbi.nlm.nih.gov/books/NBK537331/
BioGenix Peptides™ — Educational research content.

