AOD-9604 in Research: The Science Behind the Fat-Selective Growth Hormone Fragment
Research Disclaimer: This article is provided for educational and informational purposes only and summarizes published scientific literature about AOD-9604 (also described as an hGH C-terminal fragment, commonly referenced as residues 176–191 with an added N-terminal tyrosine). It is not medical advice, does not provide dosing guidance, and is not intended to diagnose, treat, cure, or prevent any disease. Any compounds mentioned are discussed strictly in a research context.
Introduction
AOD-9604 is a synthetic peptide derived from the C-terminus of human growth hormone (hGH). It was developed to preserve the lipolytic (fat-mobilizing) signaling attributed to growth hormone while avoiding classic GH effects (e.g., growth signaling via the GH receptor/IGF-1 axis and adverse glycemic changes seen with pharmacologic GH exposure). Preclinical research supports selective metabolic actions in adipose-related pathways, but human efficacy outcomes have been mixed and development for obesity indications was ultimately discontinued.
Chapter 1 — What Exactly Is AOD-9604?
1.1 Origin: a “lipolytic domain” concept
Growth hormone has long been associated with fat mass reduction and changes in lipid metabolism. The AOD-9604 development program attempted to isolate a short peptide segment of hGH thought to drive lipolysis without triggering GH receptor–mediated growth signaling. In animal work, AOD-9604 did not appear to compete for the GH receptor and showed a different metabolic side-effect profile than full-length hGH (notably less disruption of glucose/insulin parameters in the models studied).
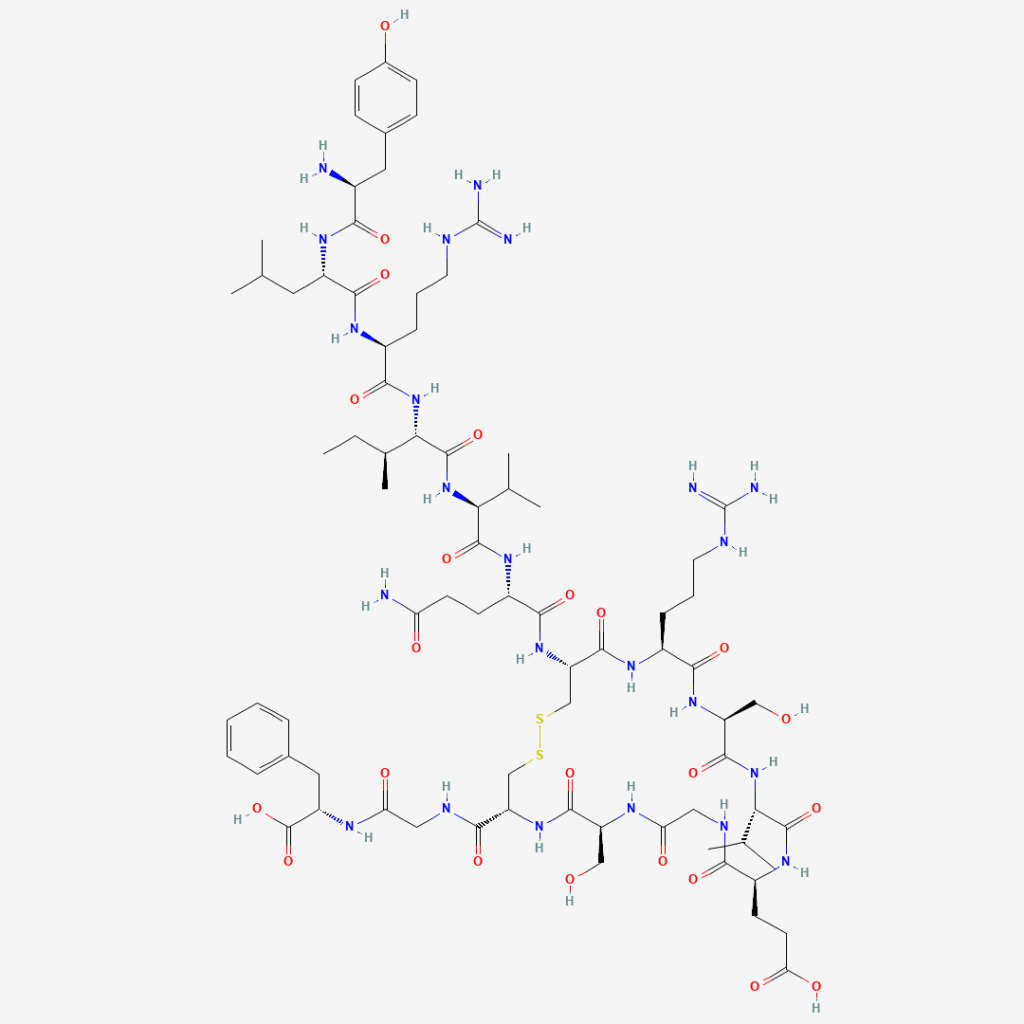
1.2 Composition and regulatory-science considerations
FDA meeting materials describing compounded drug considerations note AOD-9604 as a hexadecapeptide derived from a GH fragment (with an added N-terminal tyrosine) and highlight general peptide-development challenges such as immunogenicity potential, aggregation, peptide-related impurities, and characterization complexity.
In simple terms: AOD-9604 is a short piece of growth hormone designed to keep “fat-mobilizing” signals while avoiding “growth hormone–like” side effects. It’s still a peptide, so purity, stability, and immune reactions remain important research concerns.
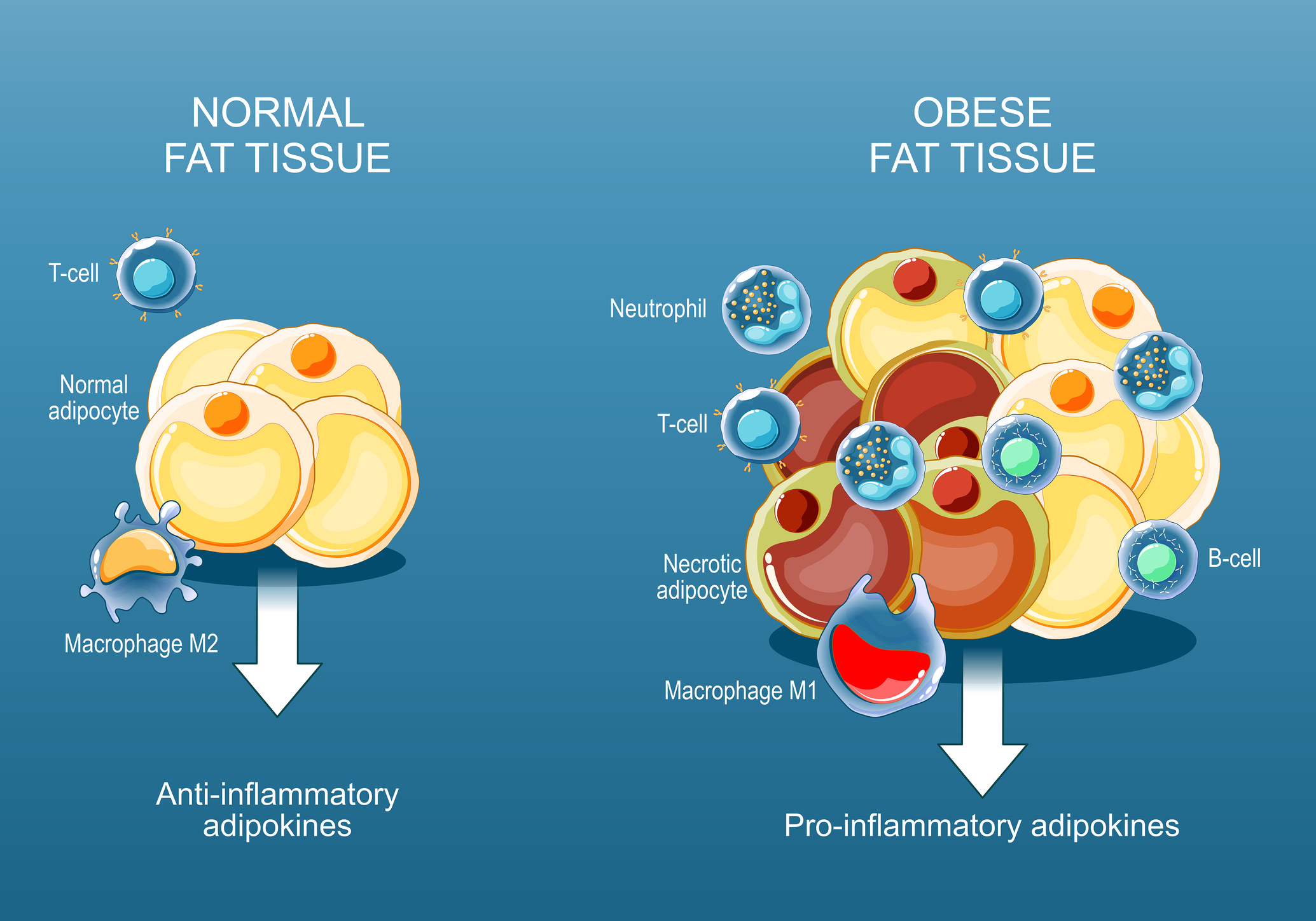
Chapter 2 — Mechanism: How Could a GH Fragment Affect Fat Tissue?
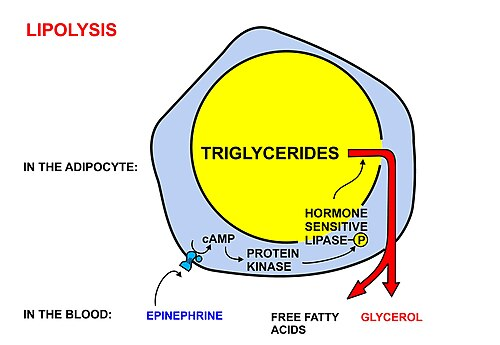
2.1 Lipolysis vs lipogenesis: the research target
“Lipolysis” refers to triglyceride breakdown in adipocytes, releasing fatty acids and glycerol. “Lipogenesis” refers to fat storage processes. The AOD-9604 program centered on selective stimulation of fat mobilization and oxidation while minimizing endocrine growth signaling.
2.2 Beta-adrenergic pathway interaction (β3-AR emphasis)
In obese mouse models, AOD-9604 and hGH both reduced body weight and fat mass, with mechanistic data consistent with interaction in the β-adrenergic pathway, including a prominent role for β3-adrenergic receptors in adipose tissue. Studies in β3-AR knockout mice were used to probe whether the weight/fat effects depended on this signaling route.
2.3 Fat oxidation signals and metabolic readouts in vivo
Preclinical studies reported increased indices of in vivo fat oxidation and increased plasma glycerol (a common lipolysis-associated marker). Importantly, in those animal studies AOD-9604 was described as not producing the same hyperglycemia/insulin secretion effects reported with hGH in the model system studied.
In simple terms: The “scientific idea” is that AOD-9604 nudges fat cells toward breaking down stored fat and burning more fat—possibly by working alongside the same adrenaline-style pathways your body uses during activity and cold exposure.
Chapter 3 — Preclinical Evidence: What Do Animal Models Actually Show?
3.1 Obese mouse models: body weight, fat mass, and pathway dependence
In obese mice, chronic administration of AOD-9604 was associated with reductions in body weight and body fat, and mechanistic work explored β-adrenergic pathway involvement (particularly β3-AR). These studies were foundational in framing AOD-9604 as a “lipolytic fragment” distinct from full-length hGH.
3.2 Oxidation and lipolysis markers
Additional animal work reported increased in vivo fat oxidation and increased plasma glycerol, interpreted as supportive of enhanced fat mobilization. The same publications contrasted AOD-9604 with hGH by emphasizing the lack of certain GH-like endocrine effects in the studied models (e.g., less adverse glycemic signaling).
3.3 Translation limits: what animal success does (and does not) mean
Even when animal outcomes are directionally consistent, translation depends on dosing exposure, route, peptide stability, receptor/pathway conservation across species, baseline metabolic state (lean vs obese), and the human trial context (diet/exercise programs can mask small drug effects). This becomes critical when interpreting later clinical findings.
In simple terms: In obese animals, AOD-9604 often looks like it helps reduce fat and increase fat-burning signals. But animals aren’t humans—what works in mice can shrink or disappear when tested in large human trials.
Chapter 4 — Human Clinical Research: What Was Explored in Trials
4.1 Early human studies and metabolic observations
Human research programs evaluating AOD-9604 included randomized, placebo-controlled study designs intended to assess metabolic signaling, tolerability, and endocrine effects. In shorter-duration trials (approximately 12 weeks), secondary literature reports that participants receiving AOD-9604 demonstrated favorable trends in body composition–related endpoints compared with placebo in certain study contexts.
In parallel, safety-focused publications summarizing multiple randomized, double-blind, placebo-controlled trials emphasized that AOD-9604 was generally well tolerated. Importantly, these studies reported no significant effects on serum IGF-1 levels or glucose tolerance, supporting the concept that AOD-9604 does not act through classic growth hormone or insulin-disruptive pathways.
4.2 Study design goals and research focus
Later-stage clinical investigations expanded study duration and participant numbers to further characterize metabolic signaling, safety margins, and consistency across populations. These trials were designed within comprehensive lifestyle-controlled frameworks, often incorporating standardized dietary guidance and physical activity protocols to minimize confounding metabolic variables.
Such designs are common in metabolic research and are intended to isolate biochemical signaling effects rather than maximize visible outcome differences. As a result, these studies contributed valuable data regarding tolerability, endocrine neutrality, and pathway specificity, even when changes in single endpoints such as scale weight were modest.
4.3 Interpreting human data in metabolic peptide research
In peptide-based metabolic research, outcome interpretation depends heavily on endpoint selection and study structure. Body weight alone is an inherently variable measure and does not always reflect changes in fat oxidation, adipocyte signaling, or substrate utilization. Factors such as baseline metabolic health, adrenergic sensitivity, and study-controlled lifestyle interventions can meaningfully influence observed results.
Collectively, the human research literature on AOD-9604 helped clarify its safety profile, mechanism selectivity, and metabolic signaling characteristics, informing subsequent scientific understanding of growth hormone–derived peptide fragments.
In simple terms: Human studies of AOD-9604 were designed to understand how it behaves in the body — especially its safety and how it differs from growth hormone. Across trials, it showed good tolerability and avoided classic growth hormone effects, helping researchers better understand how targeted peptide fragments can influence metabolic pathways.

Chapter 5 — Safety Profile: What Do Published Human Summaries Emphasize?
5.1 IGF-1 and “GH-like” endocrine effects
A key question was whether AOD-9604 would raise IGF-1 like growth hormone. A safety/tolerability summary paper reports no effect on serum IGF-1 across the evaluated trials, supporting the concept that AOD-9604 does not act via IGF-1 in the same way as GH therapy.
5.2 Glucose tolerance and insulin resistance
The same clinical safety summary reports no negative effect on carbohydrate metabolism in the trial settings assessed (including oral glucose tolerance testing), contrasted against known GH-associated glycemic risks.
5.3 Immunogenicity and antibodies
Because peptides can trigger anti-drug antibodies, immunogenicity was assessed in portions of the program. The safety summary reports no detectable anti-AOD-9604 antibodies in tested patients. FDA materials also highlight immunogenicity and aggregation as general peptide concerns—relevant for any research program handling peptides.
In simple terms: The published human summaries generally describe AOD-9604 as well tolerated, without the classic “growth hormone” lab changes (like higher IGF-1) or obvious glucose-worsening in the trial conditions reported.
Chapter 6 — Beyond Fat: Joint & Cartilage Research (Preclinical)
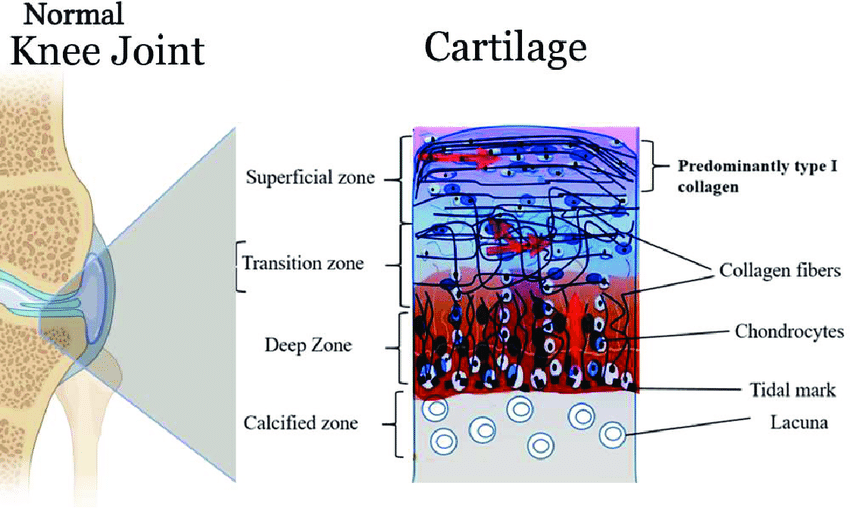
6.1 Intra-articular AOD-9604 in an osteoarthritis rabbit model
Separate from metabolic research, AOD-9604 has been studied in cartilage contexts. In a collagenase-induced rabbit knee osteoarthritis model, intra-articular AOD-9604 injections were reported to enhance cartilage regeneration, and AOD-9604 combined with hyaluronic acid was described as more effective than either alone in that model.
6.2 What this does (and does not) imply
These findings are preclinical and route-specific (intra-articular), so they should not be conflated with systemic metabolic claims. The value here is hypothesis generation: GH-fragment peptides may have tissue-level signaling effects that are context-dependent.
In simple terms: Separate animal research suggests AOD-9604 might influence cartilage repair signals when injected into the joint—interesting science, but not proof of human joint benefits.
Chapter 7 — Practical Research Considerations: Purity, Aggregation, and Study Design
7.1 Peptide characterization matters
Peptides can self-associate, aggregate, and generate complex impurity profiles (e.g., truncations, oxidations, deamidations). FDA compounding-related documents specifically call out these categories as higher-scrutiny issues for peptides (even before you get to efficacy questions).
7.2 Outcome measures that “see” mechanism
If the hypothesized action is adipose lipolysis/fat oxidation, weight alone may be a blunt instrument. Mechanism-aligned readouts (in appropriate research settings) can include changes in fat mass (imaging), substrate oxidation, glycerol/FFA kinetics, and adipose gene expression signatures tied to adrenergic sensitivity—while acknowledging that these are research endpoints and not clinical claims.
7.3 Why “no effect” can still be informative
The arc of AOD-9604 illustrates a common translational pattern: a plausible mechanism + encouraging animal data + good tolerability can still yield modest or inconsistent human efficacy. That outcome can refine future peptide design (delivery, stability, target selection) rather than “disprove biology.”
In simple terms: With peptides, quality and study design are everything. If the biology is subtle, big lifestyle changes in the trial can hide small effects—and measuring the wrong outcome can make a real mechanism look like “nothing happened.”
Conclusion
AOD-9604 remains an instructive example in peptide translational science: it was engineered to isolate a specific GH-associated metabolic effect, showed supportive preclinical signals and a generally favorable tolerability profile in summarized human trials, yet failed to demonstrate consistent, scalable weight-loss efficacy in later-stage development reported by secondary sources. Independently, cartilage-focused animal work suggests GH-fragment peptides may have broader tissue signaling roles worthy of careful, evidence-led investigation.
Peer-Reviewed References
- Heffernan MA, Thorburn AW, Fam BC, Summers R, Conway-Campbell BL, Waters MJ, et al. The effects of human GH and its lipolytic fragment (AOD9604) on lipid metabolism following chronic treatment in obese mice and β3-AR knock-out mice. Endocrinology. 2001;142(12):5182–9. PubMed | Journal
- Heffernan MA, Summers R, Thorburn AW, Fam BC, Conway-Campbell BL, Waters MJ, et al. Increase of fat oxidation and weight loss in obese mice treated with a novel fragment of human growth hormone. (Journal details as indexed in PubMed). 2001. PubMed
- Stier H, Bischoff SC, Otte JM. Safety and tolerability of the hexadecapeptide AOD9604 in humans: a review of randomized, double-blind, placebo-controlled trials. J Endocrinol Metab. 2013. Journal
- Moré MI, Stier H. Safety and metabolism of AOD9604: summary of clinical and metabolic findings across human studies. J Endocrinol Metab. 2014. Journal (PDF view)
- Misra M, et al. Obesity pharmacotherapy: current perspectives and future directions (includes discussion of AOD-9604 trial outcomes and discontinuation). Diabetes Metab Syndr Obes. 2013. Full text (PMC)
- Kwon DR, Park GY. Effect of intra-articular injection of AOD9604 with or without hyaluronic acid in rabbit osteoarthritis model. Ann Clin Lab Sci. 2015;45(4):426–32. PubMed
- U.S. Food and Drug Administration. Pharmacy Compounding Advisory Committee meeting materials (mentions AOD-9604 peptide characterization and immunogenicity/aggregation considerations). 2024 Dec 4. FDA PDF
Note: Some early AOD-9604 efficacy trials are referenced in secondary sources (reviews and safety summaries) but may not be easily retrievable as full peer-reviewed manuscripts via open databases. Where primary trial manuscripts are not openly accessible, this article cites the most direct, publicly available scientific sources and clearly labels secondary reporting.